I wanted to write this post to discuss an interesting discussion going on with regards to evolutionary basis of human complex diseases. It stems from our paper that looked into the evolution of skin barrier related gene, called fillagrin (FLG).
FLG stood out for four conceivable reasons. See this review for more.
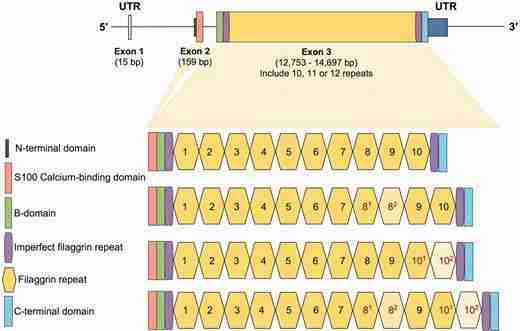
(i) It is huge. With more than 10kb in size (i.e., coding sequence), it is one of the biggest genes in the genome.
(ii) It is mostly made up of big (1kb) exonic repeats the number of which vary among individuals and probably among species. We, as a lab, are suckers for structural variation as some may know.
(iii) Variations within FLG were found to be the primary non-immune underlying factor for atopic dermatitis (eczema). And it is one of the genes that is involved in skin-barrier architecture, which is one of the most distinctive feature of humans as compared to other primates.
(iv) FLG harbors unusually high number of loss of function variants, some of which are very common in human populations.
We found FLG serendipitously. After publishing a paper on exonic repeats on a smaller gene, we searched the genome for other genes with similar subsonic copy number variation. One of our hits was FLG, emerging from the genome like a leviathan that it is. So, naturally, we wanted to understand its evolutionary history.
The first thing we noticed about FLG is that it is dying. It seems to be hit hundreds of loss of function mutations so much so that almost X number of people, independent of where they come from, carry non functioning versions of the gene. We then asked whether such loss of function is adaptive, i.e., may be there is an advantage to losing the function of this gene in certain settings as suggested by previous work.
But, in our paper, we found no signatures of such adaptive events.
Adaptive variants in humans are a complex and diverse bunch, and there is amazing discussion about how to best approach finding them. (e.g., this recent and succinct review). As such, it is possible that we did not consider a complex scenario where FLG loss of function is indeed adaptive. But, at this point, for the scenarios we did consider, there is simply no evidence for backing up adaptation. Within that context, I was surprised that there is strong enough sentiment about adaptive role of loss of function of FLG (or any other gene) that a direct response to our study was published.
The reason that I am writing this post is not to respond to our critiques. I think those who are interested can read the paper and the response. It is an interesting discussion. I wanted, instead, to take this opportunity to highlight two very relevant and general issues with regards to studying evolution of medically relevant loci:
(i) Ascertainment bias: Most of our knowledge about associations between genetic variation and diseases comes from studies of European diseases among European patients, focusing on variations common in people with European descent. This represent a small fraction of human history and variation. As such, making claims of adaptation using just European samples without a broader comparative analyses is problematic. This issue was nicely covered in multiple studies – e.g., Here, here and here.
(ii) The null hypothesis in modern evolutionary theory is neutrality: It is important to note that the onus of providing evidence remains on those who claim adaptation, as neutrality (i.e., no selection/adaptation, but random drift) is the main evolutionary force that shape genomic variation, This is the the overarching null hypothesis of modern evolutionary biology. Adaptation is murder, and a gene is innocent (neutral) until proven guilty. And the relevance to medical genetics is simple. Most of the disease causing variants are there because of bad luck.
With that, I am delighted that we, as the academic community is committed to such lively discussions. After all, these are, at the core, attempts to understand what makes us human.
